Intermolecular forces
Van der Waals (London Dispersion) Forces
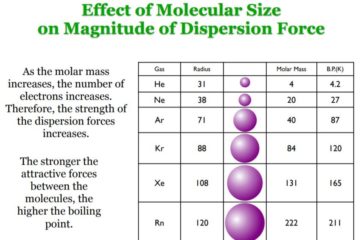
Van der Waals forces Another set of intermolecular interactions is the Van der Waals forces, which are primarily associated with non-polar molecules. They arise because of short-lived induced dipole moments which occur even in non-polar molecules. These are also sometimes referred to as London Dispersion Forces. The repulsive forces between Read more…