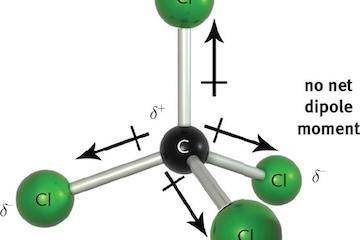
Van der Waals forces
Another set of intermolecular interactions is the Van der Waals forces, which are primarily associated with non-polar molecules. They arise because of short-lived induced dipole moments which occur even in non-polar molecules. These are also sometimes referred to as London Dispersion Forces. The repulsive forces between electrons of two or more adjacent molecules can cause their electron density to redistribute when they get close together, such that they temporarily become slightly polar, during which time they can interact with one another in a similar manner to dipole-dipole interactions. In principle, they could also interact electrostatically with molecules with permanent dipoles in this way. The term Van der Waals forces is sometimes (but not always) used to include these induced dipole-permanent dipole interactions, but not always. However, it always includes the London forces.
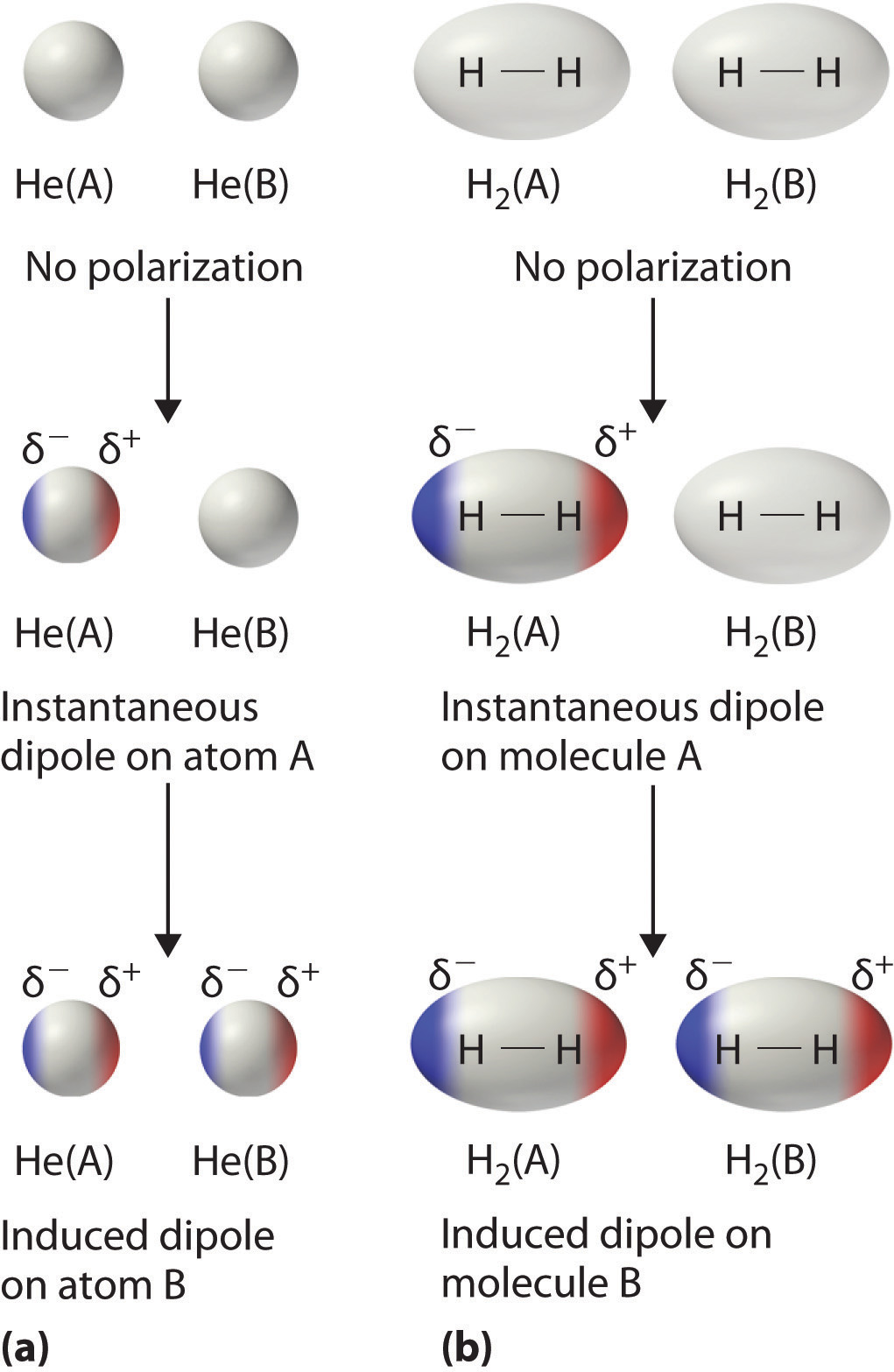
Image c/o Libre Texts
One example in which these dispersion forces become particularly important is their effects on the boiling points of hydrocarbons and other non-polar molecules. For anyone unfamiliar (or just rusty on O-chem terminology), the term hydrocarbon simply refers to a compound composed solely of carbon and hydrogen, an alkane is a hydrocarbon containing only single bonds, an alkene is a hydrocarbon containing at least one double bond, and an alkyne is a hydrocarbon containing at least one triple bond.
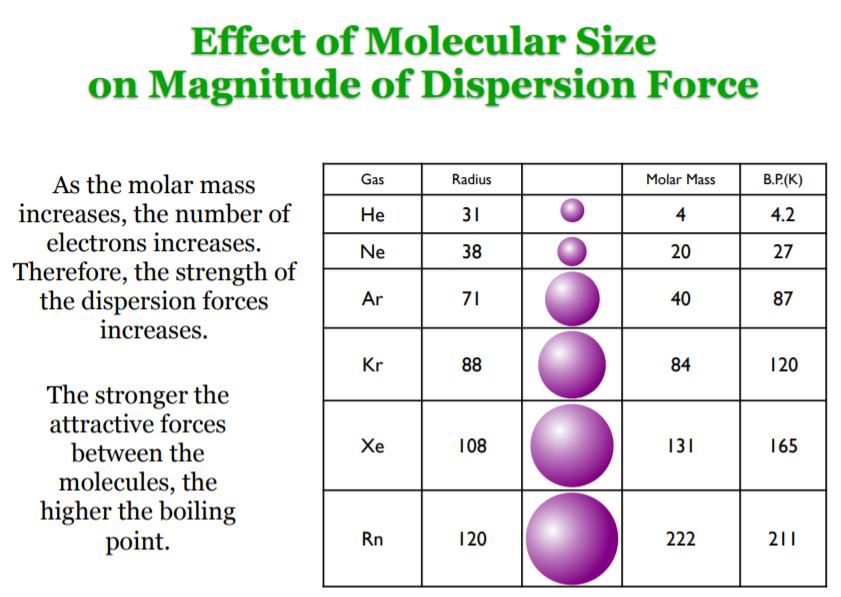
Namely, the effects of dispersion interactions on a molecule’s boiling point depend on the magnitude of the induced dipole moments, which in turn depends on the polarizability of its electrons, the volume of its electron clouds, and the size and shape of the molecule.
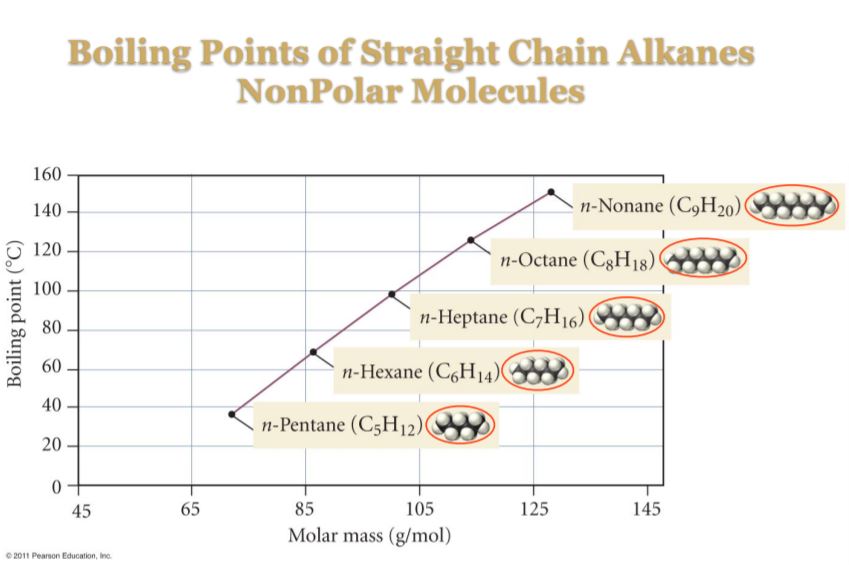
For instance, a larger molar mass corresponds to a greater number of electrons and a greater electron cloud volume, which in turn corresponds to a greater polarizability, and therefore larger induced dipole moments and greater attractive forces. All other things equal, this in turn corresponds to higher boiling points.
Moreover, larger hydrocarbons also tend to have more surface area than smaller ones, and thus undergo more surface to surface contact, which means they experience these interactions to a greater extent.
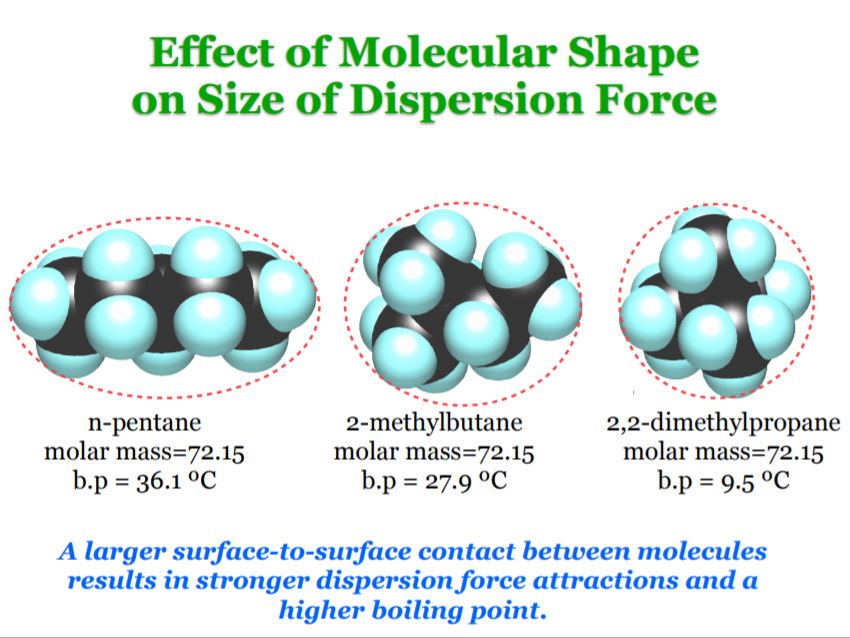
In addition to size, shape also matters. For example, long and/or flat molecules tend to have more surface area relative to their mass than more spherically shaped ones of comparable mass, which again means greater surface to surface contact between neighboring molecules, which again corresponds to stronger interactions, and therefore higher boiling points. Consequently, straight chain hydrocarbons tend to have higher boiling points than more branched isomers with the same chemical formula. An Example of this is depicted in the image below.
Pentane, 2-Methylbutane, and 2,2-Dimethylpropane are examples of what are known as constitutional isomers. That just means that they have the same chemical formula (C5H12 in this case), but with different connectivity. Pentane is a straight chain alkane, whereas 2,2-dimethylpropane is highly branched in structure, and 2-methylbutane is in between. Similarly, Pentane has the highest boiling point, 2,2-dimethylpropane has the lowest, and 2-methylbutane is in between.
So, knowing all this, you should in principle be able to estimate the relative boiling points of two comparable molecules based on their composition and structure without having to ascertain its actual numeric value via laboratory experiment. It gets more difficult to do this when these properties “compete” with one another, but it’s relatively straight forward when they are “stacked” in favor of one over the other. Plus, being able to quickly “ballpark” certain properties without explicit experiment or detailed calculation is generally a useful skill to have in science.
Return to Table of Contents:
Go to previous section: Hydrogen bonding.
Go to Credible Hulk homepage.
References
concepts, C., bond, C., & forces, I. (2017). How do atoms form covalent bond? – Core Concepts in Chemistry. Core Concepts in Chemistry. Retrieved 28 December 2018, from https://masterconceptsinchemistry.com/index.php/2017/10/05/hows-do-atoms-form-covalent-bond/
Metallic Bonding. (2013). Chemistry LibreTexts. Retrieved 28 December 2018, from https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Fundamentals_of_Chemical_Bonding/Metallic_Bonding
concepts, C., bond, C., & forces, I. (2017). How do atoms form an ionic bond? – Core Concepts in Chemistry. Core Concepts in Chemistry. Retrieved 28 December 2018, from https://masterconceptsinchemistry.com/index.php/2017/10/05/hows-an-ionic-bond-formed/
(2018). Web.mit.edu. Retrieved 7 July 2018, from http://web.mit.edu/viz/EM/visualizations/coursenotes/modules/guide02.pdf
CODATA Value: electric constant . (2018). Physics.nist.gov. Retrieved 7 July 2018, from https://physics.nist.gov/cgi-bin/cuu/Value?ep0
Bevelacqua, P. (2018). Gauss’ Law for Electric Fields. Maxwells-equations.com. Retrieved 29 December 2018, from http://www.maxwells-equations.com/gauss/law.php
8.2: Ionic Bonding. (2014). Chemistry LibreTexts. Retrieved 29 December 2018, from https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/08._Basic_Concepts_of_Chemical_Bonding/8.2%3A_Ionic_Bonding
Electronegativity. (2013). Chemistry LibreTexts. Retrieved 7 July 2018, from https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Electronegativity
Dipole Moments. (2013). Chemistry LibreTexts. Retrieved 7 July 2018, from https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Dipole_Moments
National Center for Biotechnology Information. PubChem Compound Database; CID=5943, https://pubchem.ncbi.nlm.nih.gov/compound/5943 (accessed July 7, 2018).
Polar and Nonpolar Covalent Bonds: Definitions and Examples – Video & Lesson Transcript | Study.com. (2018). Study.com. Retrieved 27 December 2018, from https://study.com/academy/lesson/polar-and-nonpolar-covalent-bonds-definitions-and-examples.html
Ionic and Covalent Binding – Problems. (2018). Chemistry.mcmaster.ca. Retrieved 27 December 2018, from https://www.chemistry.mcmaster.ca/esam/Chapter_7/problems.html
Klein, Organic Chemistry. (2015). Google Books. Retrieved 27 December 2018, from https://goo.gl/uc5Vio
Definitions: Constitutional Isomers. (2018). Chemeddl.org. Retrieved 27 December 2018, from http://www.chemeddl.org/resources/stereochem/definitions4.htm
CH2710 – Org Chem I | Professor Katz’s Chemistry Pages. (2018). Profkatz.com. Retrieved 27 December 2018, from http://profkatz.com/courses/?page_id=19