Ion-Dipole Interactions and Hydrophilicity
Ion-dipole forces are generally stronger than dipole-dipole interactions because at least one of their participants is an ion, which means it has a net electric charge, whereas dipole-dipole forces involve polar molecules with no net electric charge. These interactions explain why ionic compounds tend to be very soluble. They dissociate in water. Take salts for example (i.e. NaCl, KCl etc).
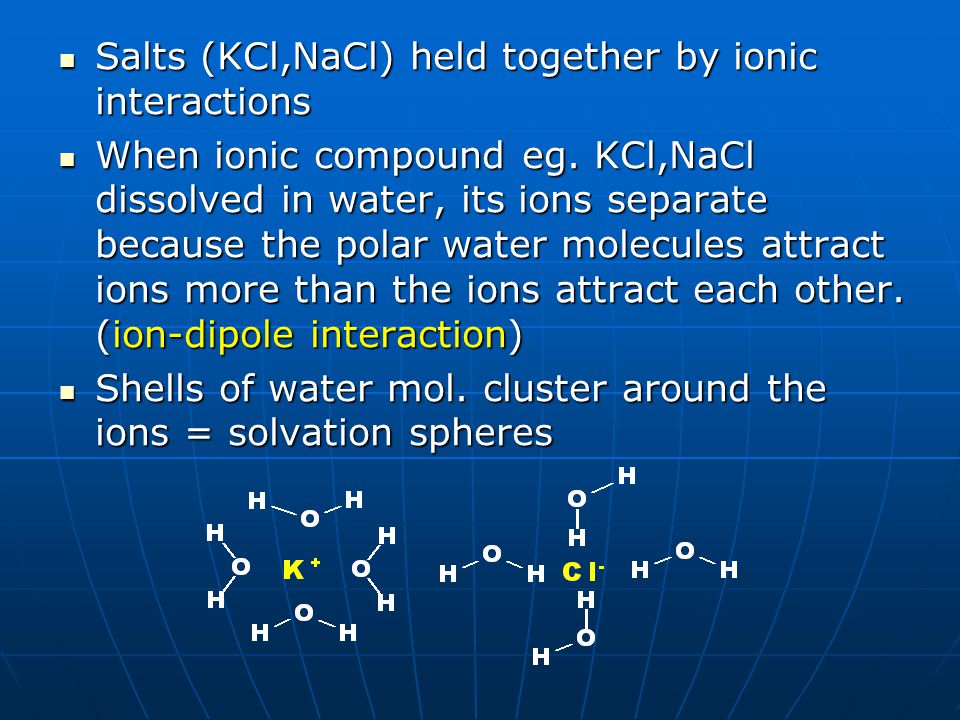
Ion-dipole interactions and solubility (Image Source):
The same is true of polar substances dissolving in water or other polar solvents. That is to say, polar and ionic species both tend to be soluble in polar solvents. In contrast, non-polar substances tend to dissolve in non-polar solvents (albeit for different reasons which I won’t go into now), hence the term “like dissolves like.”
When a substance has a strong affinity for water, meaning that it is attracted to, mixes with, and/or is dissolved by water, it is said to be hydrophilic (water loving). In contrast, a substance that is repelled by and/or doesn’t mix well in water is said to be hydrophobic (water fearing). In a nutshell, ions and polar molecules tend to be hydrophilic, whereas non-polar substances tend to be hydrophobic.
There’s a bit more to it than that, and I hope to cover the concepts of hydrophilicity and hydrophobicity in another post. For now, however, suffice it to say that the negatively charged anions interact with the partially positive hydrogens of the H2O molecules, whereas the positively charged cations interact with the partially negative oxygens, thereby minimizing the potential energy of the solution. Similarly, the partially positive parts of polar substances interact attractively with the partially negative parts of the water or other polar solvent, while the partially negative parts of the solute interact with the partially positive parts of the solvent.
Return to Table of Contents:
Go to next section: Hydrogen bonding.
Go to previous section: Dipole-dipole interactions.