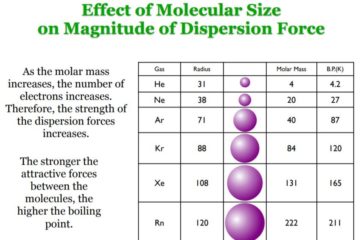
Ion-ion interactions
Recall that ions are atoms or molecules that have lost or gained one or more electrons with respect to their electrically neutral counter-parts, such that they have a net electric charge. As a result, two or more ions can exert electrostatic forces on each other. This is the principle in operation in the case of ionic compounds, such as sodium chloride (NaCl aka table salt), which typically involves a metal and non-metal ions of opposite charge. In the example of NaCl, the positively charged sodium cation is electrostatically attracted to the negatively charged chloride anion. As the two ions get closer, the Coulomb force between them get stronger.
However, ions do have structure, with electrons surrounding a positively charged nucleus, and they occupy space. Therefore, they can’t get infinitely close. All bonds release energy when formed and require an input of energy to break.
Consequently, there exists an intermolecular distance which minimizes the energy of the two-ion system with respect to the attractive interactions of their net opposite charges as well as the repulsive interactions between the electrons of neighboring ions, and thus maximizes the amount of energy released by their union.
The intermolecular distance at which this occurs is the distance at which the attractive and repulsive forces exactly balance out, and should equal the experimentally measured bond distance for the ionic bond under examination. Below is the potential energy diagram for sodium chloride as an example.
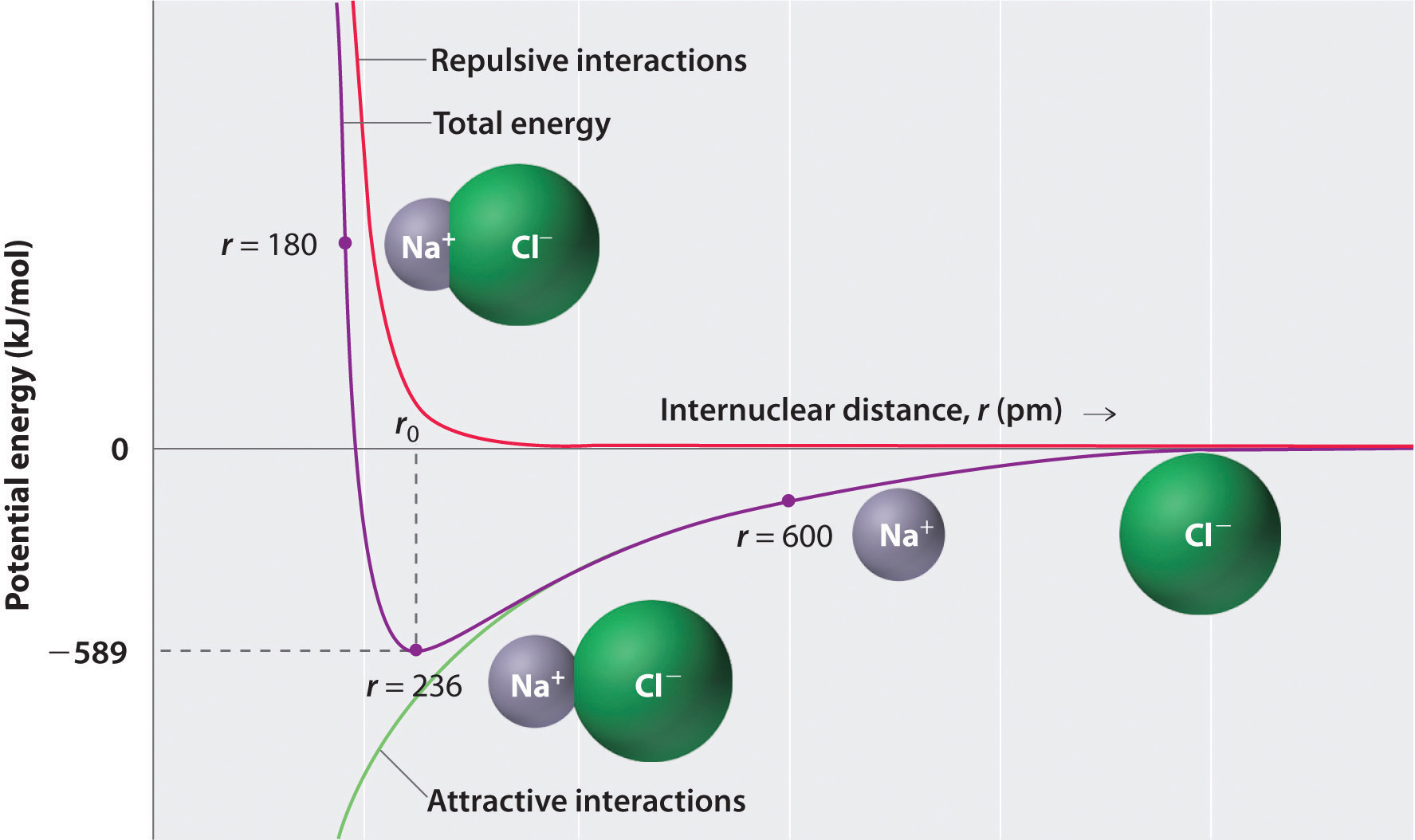
Image Source:
In contrast with non-polar covalent bonds, which involve the roughly equal sharing of electrons (generally between two non-metals), the electron density is not shared even close to equally in the case of ionic compounds. The anion generally holds more electron density, and the cation is drawn close to it via electrostatic attraction. This is due to large differences with respect to a property called electronegativity, which is a measure of how strongly a given atom draws electron density to itself.
Return to Table of Contents:
Go to next section: Dipole-dipole Interactions.
Go back to previous section: Electrostatics.